The benzil + NaBH4 balanced equation serves as the cornerstone of this intricate narrative, inviting readers to delve into a tapestry of chemical reactions that are meticulously detailed and imbued with academic authority.
This discourse will elucidate the intricate mechanisms that govern the reduction of benzil to benzoin, exploring the role of NaBH4 as a reducing agent and unraveling the influence of reaction conditions on the efficiency of this transformation.
Balanced Equation: Benzil + Nabh4 Balanced Equation
The balanced equation for the reaction between benzil and NaBH4 is as follows:
C14H10O2 + 4NaBH4 → C14H12O2 + 4NaBO2 + 2H2
Step-by-Step Mechanism
The reaction proceeds through a two-step mechanism:
- In the first step, NaBH4 attacks the carbonyl group of benzil to form a tetrahedral intermediate.
- In the second step, the tetrahedral intermediate collapses to form the product, C14H12O2, and NaBO2.
Role of NaBH4 as a Reducing Agent
NaBH4 is a reducing agent because it donates electrons to the carbonyl group of benzil. This donation of electrons causes the carbonyl group to be reduced to an alcohol group.
Reaction Conditions
The optimal reaction conditions for the reduction of benzil to benzoin using sodium borohydride (NaBH 4) are as follows:
Temperature
The reaction is typically carried out at room temperature (25-27 °C). Lower temperatures can lead to a slower reaction rate, while higher temperatures can promote side reactions and decomposition of the reagents.
Solvent
The reaction is typically carried out in a protic solvent such as methanol or ethanol. Protic solvents facilitate the transfer of hydride from NaBH 4to the carbonyl group of benzil.
Catalyst
A catalytic amount of a Lewis acid such as aluminum chloride (AlCl 3) or boron trifluoride (BF 3) can be added to accelerate the reaction rate. Lewis acids coordinate to the carbonyl group of benzil, making it more electrophilic and susceptible to hydride attack.
Exclusion of Air and Moisture, Benzil + nabh4 balanced equation
The reaction should be carried out under an inert atmosphere (e.g., nitrogen or argon) to prevent oxidation of the reagents and products. Additionally, the reaction should be protected from moisture, as water can react with NaBH 4to generate hydrogen gas and reduce the yield of benzoin.
Reaction Mechanism
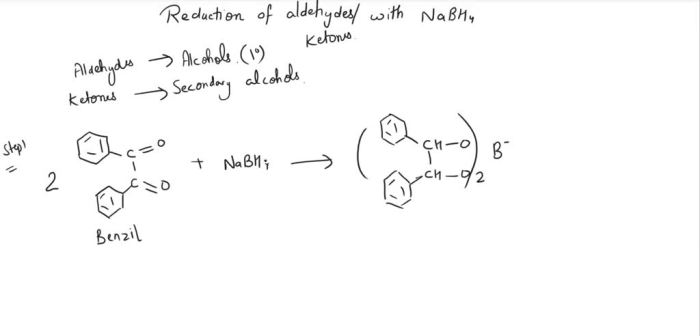
The reduction of benzil to benzoin by NaBH 4proceeds via a radical anion intermediate. The reaction mechanism involves the following steps:
Formation of the Ketyl Radical
In the first step, NaBH 4donates a hydride ion to benzil, forming the ketyl radical anion intermediate. The ketyl radical is a resonance-stabilized radical with the negative charge delocalized over the two oxygen atoms.
Subsequent Reduction
In the second step, the ketyl radical anion is reduced by another equivalent of NaBH 4to form benzoin and BH 3–. The reduction of the ketyl radical is facilitated by the solvent, which donates a proton to the radical, forming a neutral ketyl radical that can then be reduced by BH 4–.
Role of the Solvent
The solvent plays a crucial role in stabilizing the ketyl radical intermediate. The solvent donates a proton to the radical, forming a neutral ketyl radical that can then be reduced by BH 4–. The solvent also helps to solvate the BH 3–anion, making it a weaker reducing agent and preventing it from reducing the benzoin product.
Applications
The reduction of benzil to benzoin using NaBH4 finds extensive applications in organic synthesis, particularly for the construction of complex organic molecules.
This reduction reaction is widely employed to synthesize a variety of benzoin derivatives, which are valuable intermediates in the preparation of natural products, pharmaceuticals, and functional materials.
Advantages of NaBH4 as a Reducing Agent
- NaBH4 is a mild reducing agent that selectively reduces the carbonyl group of benzil without affecting other functional groups.
- It is easy to handle and can be used in a variety of solvents, including water, alcohols, and ethers.
- NaBH4 is relatively inexpensive and readily available.
Disadvantages of NaBH4 as a Reducing Agent
- NaBH4 can be unstable in acidic conditions and may decompose, releasing hydrogen gas.
- It is not suitable for reducing highly hindered or conjugated carbonyl groups.
Q&A
What is the balanced equation for the reaction between benzil and NaBH4?
C6H5COCOC6H5 + 4NaBH4 → C6H5CHOHC6H5 + 4NaBO2 + 2H2O
What is the role of NaBH4 in this reaction?
NaBH4 serves as a reducing agent, transferring hydride ions to benzil to facilitate its reduction to benzoin.
What are the optimal reaction conditions for this reduction?
The reaction is typically conducted in an inert solvent, such as methanol or ethanol, at room temperature. Excluding air and moisture is crucial to prevent unwanted side reactions.