Draw the condensed structure of an isomer of this molecule sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. Delving into the intricacies of isomerism, we embark on a journey to explore the structural variations and unique properties that define these molecular counterparts.
As we unravel the complexities of isomerism, we will decipher the nuances of structural representation, contrasting the condensed structural formulas of isomers to gain insights into their distinct arrangements. Furthermore, we will delve into the realm of isomer properties, comparing their physical and chemical characteristics to uncover the similarities and differences that govern their reactivity and stability.
1. Isomer Structural Representation

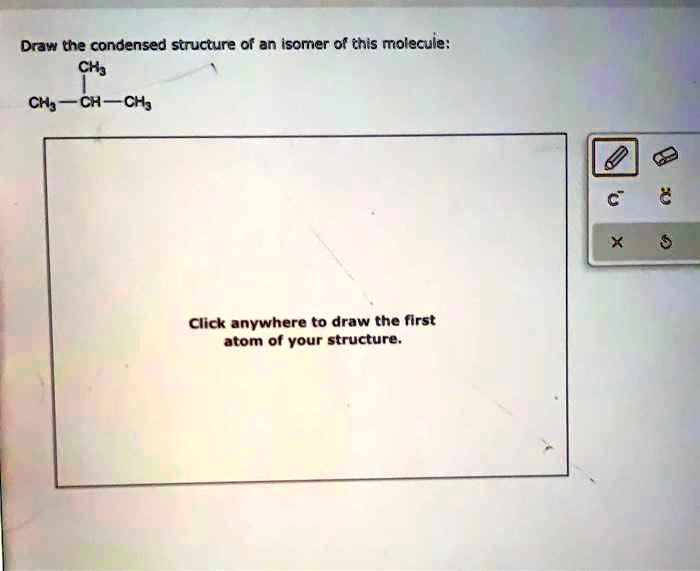
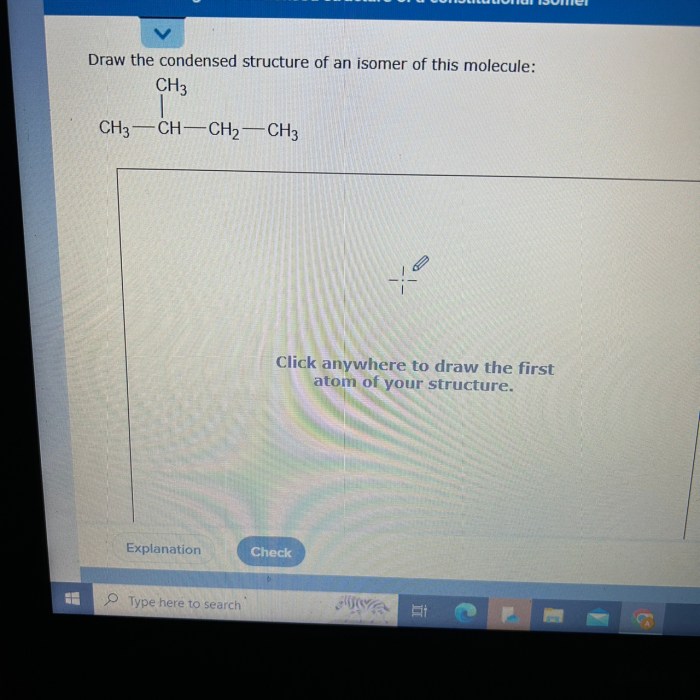
An isomer of the given molecule is a compound with the same molecular formula but a different structural arrangement of atoms. To create a condensed structural formula for an isomer, we need to rearrange the atoms within the molecule while maintaining the same number and type of atoms.
For example, an isomer of the molecule CH3CH2OH (ethanol) is CH3OCH3 (dimethyl ether). In this isomer, the hydroxyl group (-OH) and the hydrogen atom on the second carbon atom are switched, resulting in a different arrangement of atoms.
2. Isomer Properties Comparison
The physical and chemical properties of an isomer can differ from the original molecule due to the different arrangement of atoms. These differences can include:
- Boiling point: Isomers with more branching tend to have lower boiling points due to weaker intermolecular forces.
- Solubility: Isomers with polar functional groups tend to be more soluble in polar solvents, while isomers with nonpolar functional groups tend to be more soluble in nonpolar solvents.
- Reactivity: Isomers with different functional groups can have different reactivities. For example, an isomer with an alcohol group (-OH) may be more reactive towards oxidation than an isomer with an ether group (-O-).
3. Isomer Nomenclature: Draw The Condensed Structure Of An Isomer Of This Molecule
The IUPAC (International Union of Pure and Applied Chemistry) system is used to assign systematic names to isomers. The rules for naming isomers include:
- The base name of the isomer is derived from the parent hydrocarbon chain.
- The prefixes “iso-” and “neo-” are used to indicate branched isomers.
- The position of the functional group is indicated by a number.
- The suffix of the isomer indicates the type of functional group.
For example, the isomer of ethanol (CH3CH2OH) mentioned earlier would be named dimethyl ether.
4. Isomer Applications
Isomers have various applications in different fields:
- Medicine:Isomers of drugs can have different pharmacological properties, such as different potencies or side effects. For example, the isomer S-ibuprofen is more effective as a pain reliever than the R-ibuprofen isomer.
- Industry:Isomers can be used as solvents, fuels, and starting materials for chemical synthesis. For example, the isomer butane is used as a fuel in lighters and camping stoves.
- Research:Isomers can be used to study the structure-activity relationship of molecules. This information can be used to design new drugs and materials.
FAQs
What is the significance of condensed structural formulas in isomerism?
Condensed structural formulas provide a concise representation of molecular structures, highlighting the connectivity of atoms and functional groups. In the context of isomerism, they allow for the clear visualization and comparison of different structural arrangements, facilitating the identification and characterization of isomers.
How do the properties of isomers differ based on their structural variations?
Structural variations in isomers can lead to significant differences in their physical and chemical properties. These differences may manifest in variations in melting points, boiling points, solubility, reactivity, and stability. Understanding these property variations is crucial for predicting the behavior and applications of isomers.
What are the key considerations in assigning IUPAC names to isomers?
Assigning IUPAC names to isomers involves following specific rules and conventions. These rules prioritize the identification of the parent chain, functional groups, and substituents. By adhering to these guidelines, chemists ensure consistency and clarity in isomer nomenclature, enabling effective communication and unambiguous identification of these molecular counterparts.